Side effects & safety info
Do not take Otezla if you are allergic to apremilast or to any of the ingredients in Otezla.
Severe diarrhea, nausea, and vomiting have been reported with Otezla. Most of these events occurred within the first few weeks of treatment. Elderly patients and patients using Otezla with certain medications such as those used to lower blood pressure may be at higher risk. Tell your doctor if you experience severe diarrhea, nausea, or vomiting. Your doctor may lower your dose or stop your treatment with Otezla.
Some patients taking Otezla reported depression. Before starting Otezla, tell your doctor if you have had feelings of depression, suicidal thoughts, or suicidal behavior. After starting Otezla, tell your doctor if any of these symptoms, or other mood changes, develop or worsen.
Some patients taking Otezla lost body weight. Your doctor should monitor your weight regularly. If unexplained or significant weight loss occurs, your doctor will decide if you should continue taking Otezla.
You should not take certain medicines when you are taking Otezla as they may decrease its effectiveness.
Tell your doctor if you are pregnant, planning to become pregnant or planning to breastfeed.
Common side effects of Otezla
The most common side effects of Otezla were diarrhea, nausea, upper respiratory tract infection, tension headache, and headache.
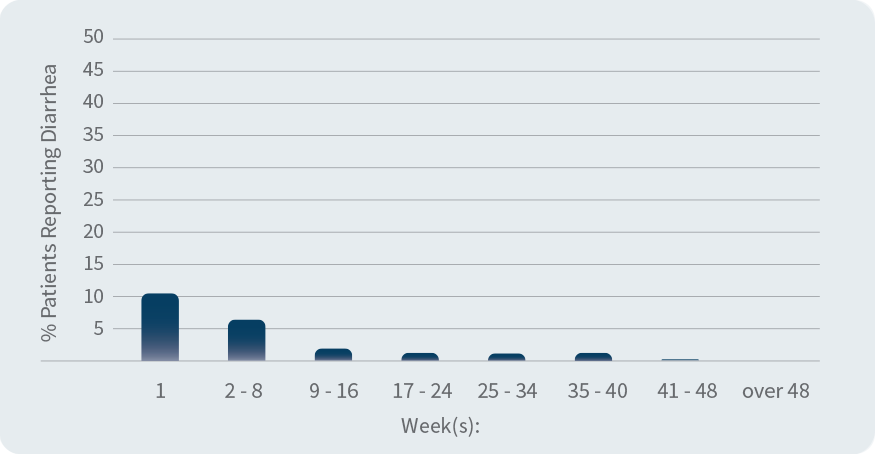
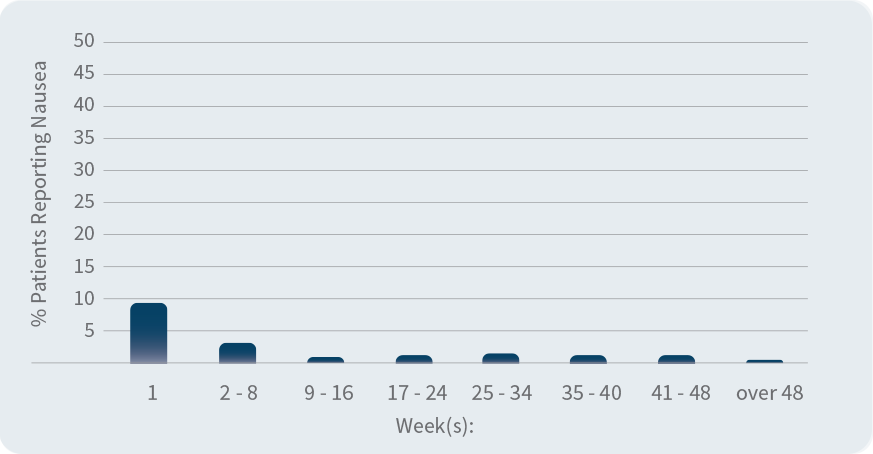
Most people reporting nausea and diarrhea did so within the first 2 weeks of treatment; these side effects tended to go away over time without stopping Otezla. Tell your doctor if any of these occur.
These are not all the possible side effects with Otezla. Tell your doctor about any side effect that bothers you or does not go away.
If you are experiencing side effects, please consult your doctor.
For more information, please see Important Safety Information to the right of this page.
Percentage of people who experienced diarrhea over time
(during clinical trials)
Percentage of people who experienced nausea over time
(during clinical trials)
Percentage of people who experienced diarrhea over time
(during clinical trials)

Otezla clinical studies involved 1,426 adults with moderate to severe plaque psoriasis who were candidates for phototherapy or systemic therapy.
Percentage of people who experienced nausea over time
(during clinical trials)

Otezla clinical studies involved 1,426 adults with moderate to severe plaque psoriasis who were candidates for phototherapy or systemic therapy.